Get the full story of the microbiome
- Importance of Microbial Diversity
- Dysbiosis and Recurrent C. difficile Infection
- Historic Microbiome Restoration Approaches
- Patient and Healthcare Burdens
Currently approved C. difficile infection (CDI) treatments are designed to address the acute infection.
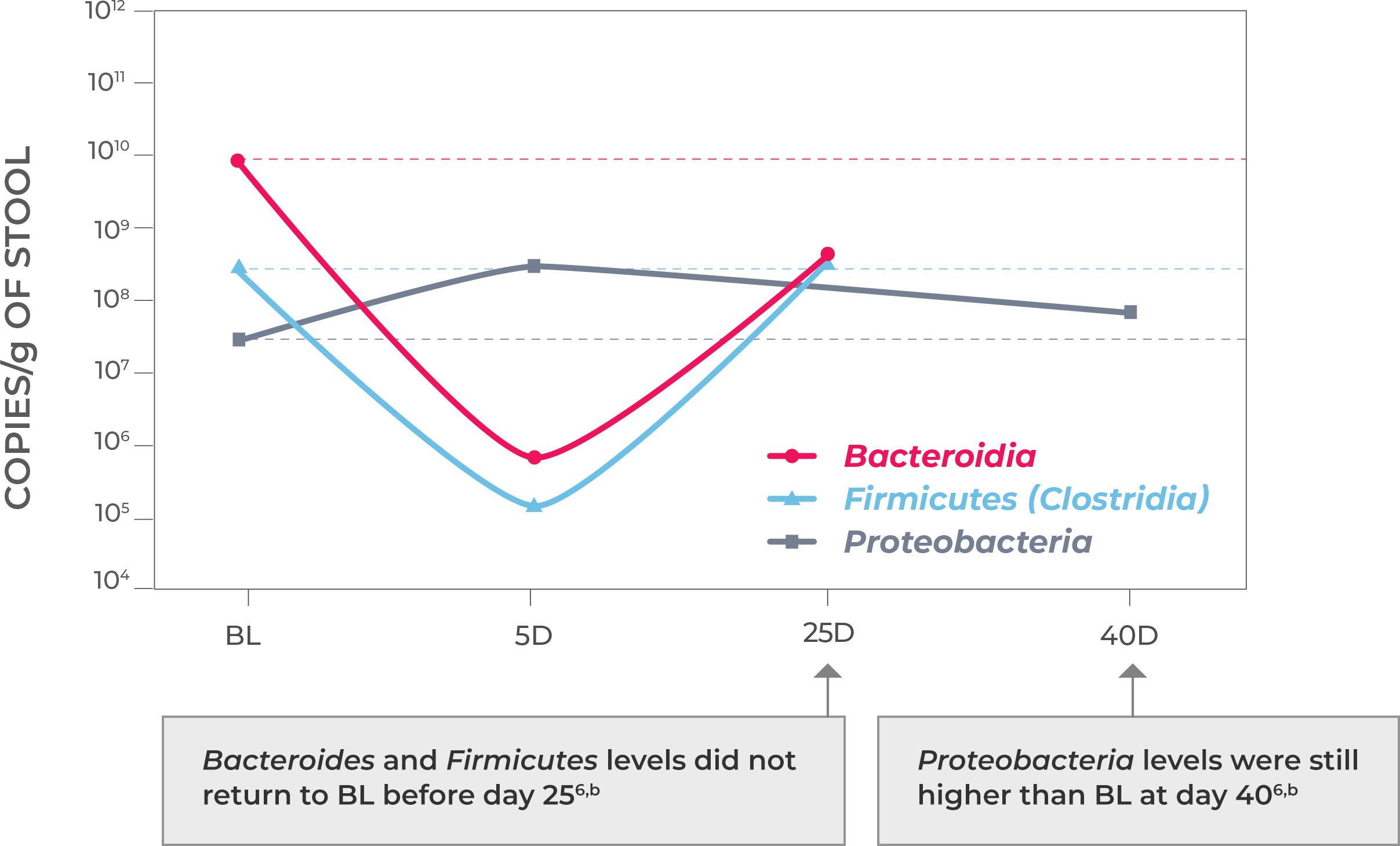
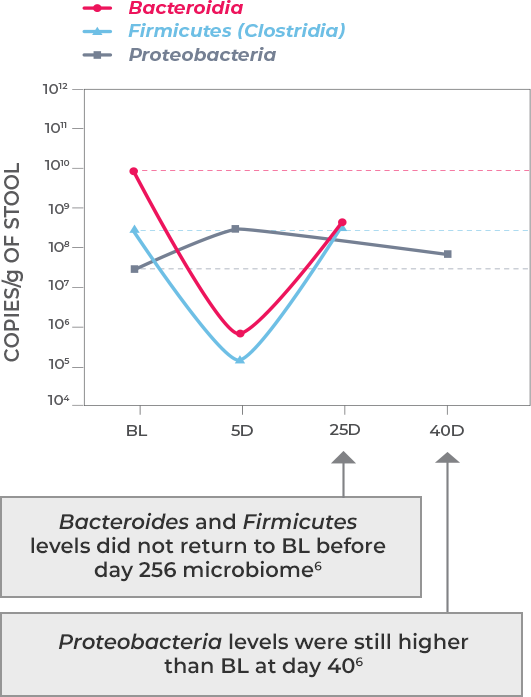
Antibiotic treatment—including vancomycin and fidaxomicin—address the acute infection but do not address (and possibly worsen) dysbiosis or restore the diverse composition of the gut microbiome. This allows a window of vulnerability for recurrence.1-3
Microbiota disruption increases the risk of CDI by providing a niche for C. difficile to flourish. Should antibiotics disrupt the intestinal microbiota, long-lasting effects may result, as well as increased risk of CDI recurrence. Longer exposure to multiple antibiotics and treatment with multiple antibiotics may also increase the risk.4,5
THE BURDEN OF CDI MAY INCREASE
WHEN THE INFECTION RECURS
Can the power of the microbiome be
unlocked to break the cycle of rCDI?
Share this with colleagues!

References
- DePestel DD, Aronoff DM. Epidemiology of Clostridium difficile infection. J Pharm Pract. 2013;26(5):464-475.
- Knight CL, Surawicz CM. Clostridium difficile infection. Med Clin North Am. 2013;97(4):523-536.
- Aukes L, Fireman B, Lewis E, et al. A risk score to predict Clostridioides difficile infection. Open Forum Infect Dis. 2021;8(3):ofab052.
- Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18(suppl 6):21-27.
- Smits WK, Lyras D, Lacy DB, Wilcox MH, Kuiiper EJ. Clostridium difficile infection. Nat Rev Dis Primers. 2016;2:16020.
- Thorpe CM, Kane AV, Chang J, Tai A, Vickers RJ, Snydman DR. Enhanced preservation of the human intestinal microbiota by ridinilazole, a novel Clostridium difficile-targeting antibacterial, compared to vancomycin. PLoS One. 2018;13(8):e0199810.
- Ferdyan N, Papazyan R, Walsh D, et al. Rapid restoration of bile acid compositions after treatment with investigational microbiota-based therapeutic RBX2660 for recurrent Clostrioides difficile infection. Open Forum Infect Dis. 2020;7(suppl 1):S15-S16.
- Zinplava [prescribing information]. Whitehouse Station, NJ: Merck & Co., Inc; 2016.
- Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825-834.
- Van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407-415.
- Guh AY, Mu LG, Winston LG, et al. Trends in U.S. burden of Clostridioides difficile infection and outcomes. N Engl J Med. 2020;382(14):1320-1330.
- Feuerstadt P, Stong L, Dahdal DN, Sacks N, Lang K, Nelson WW. Healthcare resource utilization and direct medical costs associated with index and recurrent Clostridioides difficile infection: a real-world data analysis. J Med Econ. 2020;23(6):603-609.